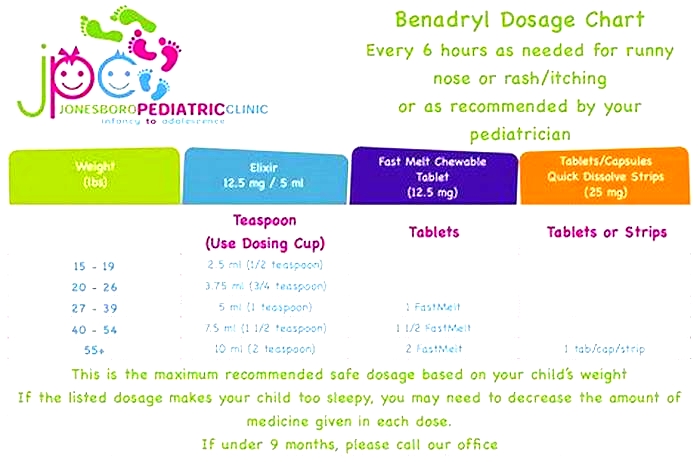
Why is Benadryl not recommended anymore

Why Is Benadryl Not Recommended Anymore
Benadryl, also known as diphenhydramine, is not recommended as the first line treatment for allergies and allergic reactions anymore for several reasons:
1. Sedative effects: Benadryl is a first-generation antihistamine that can cause drowsiness and sedation, affecting a persons ability to function normally and potentially causing impairment. This is especially concerning for tasks that require alertness, such as driving.
2. Anticholinergic side effects: Benadryl can cause dry mouth, blurred vision, constipation, and difficulty urinating. It can also cause confusion, especially in older adults, and may increase the risk of falls in the elderly.
3. Short duration of action: Benadryl has a relatively short duration of action, requiring multiple doses throughout the day to maintain its effect. This can be inconvenient for patients and may not provide adequate relief for longer-lasting allergic symptoms.
4. Newer, more effective options: There are newer, second-generation antihistamines available that are less sedating and have a longer duration of action, such as loratadine, cetirizine, and fexofenadine. These medications are generally preferred for managing allergies and allergic reactions due to their improved safety profile and efficacy.
Overall, Benadryl is still used in certain situations, such as for its sedative effects in treating insomnia or allergic reactions, but it is no longer recommended as the first-line treatment for allergies due to its side effects and the availability of more effective and safer alternatives.
Dr. Richard Young is a board certified cosmetic and reconstructive plastic surgeon
As one of the nations leading innovators in aesthetic surgery of the face, hand, breast and body, and a pioneer of reconstructive surgery and stem cell procedures, Dr. Richard Young is certified by the Board of Plastic Surgery and the Board of Otolaryngology Head and Neck Surgery.
by Richard YoungReviewed by Richard Youngapproved by Richard Young
Written by Dr Richard Young. The article was written and approved by Dr Richard Young, who specializes in plastic surgery.
The web page content is prepared to inform the visitor. The information on the page can never replace a physicians treatment or consultation. The content was prepared and published by Dr Richard Young, who is trained and specialized in plastic surgery. The content is based on the education and experience of Dr Richard Young. Copying the content is prohibited.
Dr. Richard Young
About Us
Evidence update for the treatment of anaphylaxis
Resuscitation. 2021 Jun; 163: 8696.
Evidence update for the treatment of anaphylaxis
,a,1 ,a,1 ,b ,c ,d,2 and e,2,
Amy Dodd
aSevern Deanery, UK
Anna Hughes
aSevern Deanery, UK
Nicholas Sargant
bBristol Royal Hospital for Children, Bristol, UK
Andrew F. Whyte
cUniversity Hospitals Plymouth NHS Trust, Plymouth, UK
Jasmeet Soar
dNorth Bristol NHS Trust, Bristol, UK
Paul J. Turner
eNational Heart and Lung Institute, Imperial College London, UK
aSevern Deanery, UK
bBristol Royal Hospital for Children, Bristol, UK
cUniversity Hospitals Plymouth NHS Trust, Plymouth, UK
dNorth Bristol NHS Trust, Bristol, UK
eNational Heart and Lung Institute, Imperial College London, UK
Corresponding author at: National Heart and Lung Institute, Imperial College London, Norfolk Place, London W2 1PG, UK.
[email protected]1Joint first authors.
2Joint last authors.
Received 2021 Jan 24; Revised 2021 Mar 10; Accepted 2021 Apr 12.
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Abstract
The Resuscitation Council UK has updated its Guideline for healthcare providers on the Emergency treatment of anaphylaxis. As part of this process, an evidence review was undertaken by the Guideline Working Group, using an internationally-accepted approach for adoption, adaptation, and de novo guideline development based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence to decision (EtD) framework, referred to as GRADE-ADOLOPMENT. A number of significant changes have been made, which will be reflected in the updated Guideline. These include: emphasis on repeating intramuscular adrenaline doses after 5min if symptoms of anaphylaxis do not resolve; corticosteroids (e.g. hydrocortisone) no longer being routinely recommended for the emergency treatment of anaphylaxis; interventions for reactions which are refractory to initial treatment with adrenaline; a recommendation against the use of antihistamines for the acute management of anaphylaxis; and guidance relating to the duration of observation following anaphylaxis, and timing of discharge.
Keywords: Adrenaline, Anaphylaxis, Antihistamine, Corticosteroids, Resuscitation
Introduction
The World Allergy Organisation (WAO) defines anaphylaxis as a serious systemic hypersensitivity reaction that is usually rapid in onset and may cause death. Severe anaphylaxis is characterized by potentially life-threatening compromise in airway, breathing and/or the circulation, and may occur without typical skin features or circulatory shock being present.1 Anaphylaxis thus lies along a spectrum of severity, ranging from mild objective breathing problems (such as mild wheezing) to circulatory shock and/or collapse (anaphylactic shock). The estimated incidence for anaphylaxis in Europe is 1.5 to 7.9 per 100,000 person-years, with a lifetime prevalence of 1 in 300.2 International guidelines concur that the first line treatment of anaphylaxis is intramuscular (IM) adrenaline,3 but there is increasing divergence between published guidelines.4 This may be due to a lack of high-certainty evidence to support treatment recommendations.5 Given the difficulties of undertaking randomised controlled trials in the management of a potentially life-threatening condition, guidelines must therefore be based on the best available research evidence, theory and expert consensus.
This evidence review was undertaken by the Anaphylaxis Working Group of the Resuscitation Council UK (RCUK), to support the 2021 update of the RCUK guidelines for the emergency treatment of anaphylaxis. The Working Group used an internationally-accepted approach for adoption, adaptation, and de novo guideline development based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence to decision (EtD) framework, referred to as GRADE-ADOLOPMENT.6 The EtD framework facilitates the use of evidence in a structured and transparent way to inform decisions in the context of clinical and public health recommendations and decisions.7The approach is outlined in . In brief, key research questions (see ) were identified from the previous RCUK guideline. The EtD framework for each question/topic, incorporating a review of existing guidelines and published systematic reviews, was independently completed by two assessors. We included international guidelines irrespective of whether they used the GRADE EtD framework (and some guidelines preceded the EtD methodology). The EtD tables were then reviewed by the Working Group, and a consensus reached as to (i) the certainty of the available evidence () and (ii) whether this supported the previous recommendation (adopted), indicated a need to update the recommendation (adapted) or develop an entirely new recommendation. The strength for each recommendation was assigned as either strong or weak (see ).8 Reasons for a weak recommendation include: the absence of high-certainty evidence; imprecision in outcome estimates; variability in the values and preferences of individuals regarding the outcomes of interventions; small benefits; applicability in all settings versus specific settings; and benefits that may not be worth the costs (including the costs of implementing the recommendation). These criteria are summarised in Table S1, supplementary material. Finally, recommendations and their evidence base were reviewed by a Consultation Panel (see acknowledgements) prior to a public consultation (via the Resuscitation Council UK website, between 23 December 2020 and 24 February 2021, resulting in 130 submissions) and finalisation by the working group.
GRADE ADOLOPMENT process.
Table 1
Identified research questions for evaluation.
| RCUK 2008 recommendation | Research question for review |
|---|---|
| Adrenaline is the most important drug for the treatment of an anaphylactic reaction. The intramuscular (IM) route for adrenaline is the route of choice for most healthcare providers. | Is adrenaline effective for the treatment of anaphylaxis? |
| What is the optimal timing of adrenaline in the treatment of anaphylaxis? | |
| What is the optimal route of adrenaline to treat anaphylaxis? | |
| Adrenaline IM dose Adults 0.5mg IM Children: the scientific basis for the recommended doses is weak. | What is the optimal dose of intramuscular adrenaline in the treatment of anaphylaxis? |
| Repeat the IM adrenaline dose if there is no improvement in the patient's condition. Further doses can be given at about 5-min intervals according to the patient's response. | Is adrenaline effective in the treatment of anaphylaxis reactions refractory to initial treatment with adrenaline? |
| Large volumes of fluid may leak from the patient's circulation during an anaphylactic reaction Give a rapid IV fluid challenge and monitor the response; give further doses as necessary. | Are intravenous fluids effective as an adjuvant treatment for anaphylaxis? |
| Antihistamines are a second line treatment for an anaphylactic reaction. The evidence to support their use is weak, but there are logical reasons for them.Before discharge from hospital all patients must be considered for anti-histamines and oral steroid therapy for up to 3 days | Are antihistamines effective in the treatment of anaphylaxis? |
| Corticosteroids may help prevent or shorten protracted reactions.Before discharge from hospital all patients must be considered for anti-histamines and oral steroid therapy for up to 3 days | Are corticosteroids effective in the treatment of anaphylaxis? |
| Consider further bronchodilator therapy with salbutamol (inhaled or IV), ipratropium (inhaled), aminophylline (IV) or magnesium (IV). | Are inhaled beta-2 agonists effective in the treatment of anaphylaxis? |
| Patients should be observed for at least 6h in a clinical area with facilities for treating life-threatening ABC problems | How long should patients be observed in hospital following anaphylaxis? |
Table 2
| Certainty of evidence | Explanation |
|---|---|
| High | We are very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect |
| Very low | We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
Table 3
Interpretation of strong and weak recommendations.8
| Implications | Strong recommendation | Weak recommendation |
|---|---|---|
| For patients | Most individuals in this situation would want the recommended course of action and only a small proportion would not. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| For clinicians | Most individuals should receive the intervention. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with his or her values and preferences. Decision aids may be useful helping individuals making decisions consistent with their values and preferences. |
| For policy makers | The recommendation can be adapted as policy in most situations. | Policymaking will require substantial debate and involvement of various stakeholders. |
The guidelines reviewed were those from: British Society for Allergy & Clinical Immunology (BSACI)9, 10; National Institute for Health and Care Excellence (NICE)11; European Academy of Allergy and Clinical Immunology (EAACI)12, 13; Australasian Society of Clinical Immunology and Allergy (ASCIA)14; Joint Task Force on Practice Parameters (JTFPP) of the American Academy of Allergy, Asthma & Immunology (AAAAI) and the American College of Allergy, Asthma and Immunology (ACAAI)15, 16; Canadian Society of Allergy and Clinical Immunology (CSACI)17; World Allergy Organisation (WAO).1, 3, 18, 19, 20, 21, 22 The EAACI 2021 updated guideline and JTFPP 2020 documents followed the GRADE EtD framework. Systematic reviews of anaphylaxis treatment (including both randomised controlled trials and observational studies) published in the last 10 years were identified by searching PubMed and Cochrane Database of Systematic Reviews.
Is adrenaline effective for the treatment of anaphylaxis?
Recommendation
We recommend adrenaline as the first line treatment for anaphylaxis (strong recommendation, moderate certainty evidence)
(adopted from RCUK 2008 and EAACI 2014 guidelines)
Rationale
International guidelines agree that adrenaline (epinephrine) is first line treatment for anaphylaxis. However, supporting evidence is limited to observational studies (case series and fatality registers) in humans, animal models, epidemiological studies, and pharmacokinetic studies in patients who might be at risk for anaphylaxis but not experiencing allergic symptoms at the time of study. The EAACI 2014 guideline concluded there is some evidence to support the use of adrenaline for the emergency management of anaphylaxis,12 while the WAO 2011 Guideline noted that the evidence base for prompt epinephrine injection in the initial treatment of anaphylaxis is stronger than the evidence base for the use of antihistamines and glucocorticoids in anaphylaxis.18 A systematic review by EAACI in 2020 only identified observational studies examining the efficacy of adrenaline and noted a high risk of bias; no eligible studies compared adrenaline with no adrenaline for critical outcomes such as mortality, or most other outcomes.23
There is little doubt that sufficient adrenaline results in symptom resolution, and that delayed administration is associated with protracted reactions, hypotension and fatal outcomes.23, 24 Fatal outcomes due to anaphylaxis are rare,25, 26 and around 80% of reactions resolve without (or despite no treatment with) adrenaline.27, 28 However, severe reactions cannot be predicted,1 thus all anaphylaxis reactions must be treated as potentially life-threatening. At least one-third of deaths due to food-induced anaphylaxis in the UK occur despite timely administration of adrenaline;29 observational studies30 and data from animal models31 indicate that this is likely due to severe reactions requiring more than one or two doses of IM adrenaline. Around 10% of anaphylaxis events demonstrate a suboptimal response to a single dose of adrenaline; most will respond to one or two further doses.32
Overall, the evidence for adrenaline to treat anaphylaxis was graded as moderate certainty () while confidence in the effect estimate is limited, data from a systematic review and meta-analysis (including 36,557 anaphylaxis events) indicates that only 2.2% (95% CI 1.14.1%) of reactions fail to respond to two doses of adrenaline.32 It was deemed very unlikely that the true effect would be substantially different from this estimate, thus under the EtD framework () the certainty was assigned as moderate. The strong recommendation for adrenaline is based on the working group placing a high value on evidence suggesting that adrenaline is the most appropriate treatment to reduce morbidity, recommendations for its use in existing anaphylaxis guidelines, and feedback from the public consultation.
Anaphylaxis may resolve but then exhibit a recrudescence several hours later in the absence of further exposure to an allergen (biphasic reaction). A systematic review and meta-analysis of 27 studies (2758 patients, 5% rate of biphasic reactions) reported no impact of adrenaline treatment on the occurrence of biphasic reactions (pooled OR 0.91, 95% CI 0.61.4).33 This is consistent with data from the European Anaphylaxis Registry (7328 patients, 5% rate of biphasic reactions; OR 0.91, 95% CI 0.711.16).34 The EAACI 2020 systematic review reported two relevant case-control studies, but could not comment on whether adrenaline prevents biphasic anaphylactic reactions because the certainty of evidence was very low.23
What is the optimal timing of adrenaline in the treatment of anaphylaxis?
Recommendation
Adrenaline should be administered early once symptoms of anaphylaxis have been recognized or suspected (weak recommendation, very low certainty evidence).
(adopted from RCUK 2008 and EAACI 2014 guidelines)
Rationale
There is a lack of high-certainty evidence to differentiate the effect of early versus delayed administration of adrenaline on clinical outcomes.23 Case series (including reports of fatal anaphylaxis) suggest that early adrenaline administration for out-of-hospital anaphylaxis is associated with improved outcomes.12 There is no evidence that pre-emptive use of adrenaline to treat mild, non-anaphylaxis reactions prevents progression to anaphylaxis.35 However, despite the lack of evidence to inform the optimal timing of administration,23 it seems reasonable to recommend adrenaline is given as soon as features of anaphylaxis are apparent; this is the consensus reflected in international guidelines.
With respect to biphasic reactions, the 2020 JTFPP identified eight retrospective case series, three of which found that delayed administration was associated with a higher rate of biphasic reaction.16 A prospective cohort study of 430 anaphylaxis reactions found that delayed administration of adrenaline (more than 30min after onset of symptoms) was associated with a higher rate of biphasic reaction (OR 3.39, 95% CI 1.1310.18).36 The 2020 JTFPP concluded that there does appear to be a trend to lower rates of biphasic reactions with earlier epinephrine administration following development of anaphylaxis.16
What is the optimal route of adrenaline to treat anaphylaxis?
Updated recommendations
1.
The intramuscular (IM) route is recommended for initial adrenaline treatment for anaphylaxis (strong recommendation, very low certainty evidence).
2.
The intravenous (IV) route is not recommended for initial management of anaphylaxis, except in the perioperative setting (as an alternative to IM adrenaline) by those skilled and experienced in its use (good practice statement).
3.
Titrate the administration of adrenaline (by any route) against clinical response (strong recommendation, very low certainty evidence).
(adapted from RCUK 2008 and EAACI 2014 guidelines, with greater emphasis on IM route and where needed, use of IV adrenaline infusion rather than IV bolus therapy)
Rationale
There are no trials comparing different routes of adrenaline administration in patients during anaphylaxis. IM adrenaline is recommended over other routes of administration for initial treatment of anaphylaxis, due to a favourable adverse event profile (including in those with cardiovascular co-morbidities).1, 12 The subcutaneous route is not recommended, on the basis of (low certainty) evidence that higher plasma adrenaline levels are achieved by the IM route;37 the available data relates to pharmacokinetic studies undertaken in patients outside the context of an allergic reaction and may be confounded by using different injection sites (thigh versus arm), in addition to different depth of injection.23 Comparing the IM to IV route, the EAACI 2020 systematic review identified a single case series (children and adults) which found that IV bolus administration was associated with a 13% increase in the incidence of adrenaline overdose and an 8% increase in the incidence of cardiovascular events compared with IM administration.38 Excessive doses of adrenaline, particularly by the IV route, can cause tachyarrhythmias, severe hypertension, myocardial infarction and stroke. Fatalities have occurred in the UK due to the inappropriate use of intravenous adrenaline to treat allergic (but non-anaphylaxis) reactions.39 Both IM and IV routes are recommended for treating perioperative anaphylaxis by experienced anaesthetists,40, 41 although international guidelines recommend IM adrenaline for first-line treatment of anaphylaxis in all settings. If cardiac arrest is imminent or has already occurred, an intravenous (or interosseous) bolus dose of adrenaline is indicated.4
Although the evidence was assessed as being of low certainty, the working group agreed with the evaluation in other guidelines that given the totality of the evidence and clinical experience over many decades a strong recommendation for the use of intramuscular adrenaline was appropriate.13 A strong recommendation for the IM route was deemed justified, as the working group placed a high value on the relative ease and safety of IM adrenaline administration by a wide variety of healthcare staff, and the current acceptance of the IM route in both clinical and non-clinical settings (including by patients for self-administration using an autoinjector device). Despite the limited evidence, we have made a strong recommendation for titrating the dose of adrenaline (as an intravenous infusion) against the clinical response, since this is routine in clinical practice to mitigate against the side effects of excessive adrenaline administration.
What is the optimal dose of intramuscular adrenaline in the treatment of anaphylaxis?
Recommendation
Intramuscular adrenaline should be administered at the doses listed in : (strong recommendation, low certainty evidence)
Table 4
Recommended doses of IM adrenaline.
| Adrenaline IM dose adults | |
| 500 micrograms (0.5mg) IM (0.5mL of 1mg/ml [1:1000] adrenaline) | |
| Adrenaline IM dose children | |
| >12 years | 500 micrograms IM (0.5mL) i.e. same as adult dose300 micrograms (0.3mL) if child is small or prepubertal |
| 612 years | 300 micrograms IM (0.3mL) |
| 6 months6 years | 150 micrograms IM (0.15mL) |
| <6 months | 100150 micrograms IM (0.10.15mL) |
(adopted from RCUK 2008 and EAACI 2014 guidelines)
Rationale
The safety and efficacy of the dosing regimen () has been established in clinical practice for over 20 years. In children, a dose of 0.01mg/kg (max 500 microgram) titrated to clinical response is recommended in international guidelines. Many guidelines (including those from EAACI, WAO and RCUK) simplify the dosing regimen to age categories, based on what is considered to be safe and practical to draw up and inject in an emergency.42 This pragmatic approach (which matches the licensed doses used for auto-injectors) seems to be effective and safe. Four small crossover RCTs have been published which compare different doses of IM adrenaline: one in children (weight 1530kg) comparing 150/300 micrograms;43 and three comparing 300/500 micrograms in teenagers44 or adults.45, 46 In all four studies, the higher dose had a more favourable absorption profile, however how this impacts on clinical response in patients with anaphylaxis is unknown. While the certainty of evidence with respect to dose is low, the working group concluded that a strong recommendation was appropriate given these doses have been widely used globally for many decades. In addition, we did not identify any new evidence to challenge current dosing recommendations.
In terms of the practicalities of IM administration, the EAACI 2020 systematic review identified one study in which untrained caregivers were more able to give adrenaline using a prefilled syringe correctly, than when using an adrenaline auto-injector (AAI) (OR 4.07, 95%CI 1.2912.86; low certainty).47 A study with radiologists found that using an AAI reduced the time to administration by an average of 70s compared to drawing up manually from an ampoule, and resulted in fewer administration errors.48 Most AAIs deliver a maximum of 300 micrograms epinephrine, while the appropriate dose in teenagers and adults is 500 micrograms. Coronial inquests have identified that the use of AAIs for anaphylaxis can therefore result in substantial underdosing, which may contribute to fatal outcomes.49, 50 A single-blinded, cross-over RCT in 12 food-allergic teenagers reported that a 500 microgram dose (given by AAI) had a more favourable pharmacokinetic and pharmacodynamic profile compared to 300 micrograms, without causing a higher rate of systemic adverse events.44 Therefore, while some settings may prefer to use an AAI to administer an initial dose of adrenaline (for speed and ease), further doses should be given by needle/syringe in order to deliver an optimal dose.
Are additional doses of adrenaline effective in the treatment of anaphylaxis reactions refractory to initial treatment with adrenaline?
Updated recommendations
1.
Subsequent doses of adrenaline should be given every 5min, titrated to clinical response, in patients whose symptoms are refractory to initial treatment (weak recommendation, very low certainty evidence).
2.
Where respiratory and/or cardiovascular features of anaphylaxis persist despite 2 appropriate doses of adrenaline (administered by IM or IV route), seek urgent expert help (e.g. from experienced critical care clinicians) to establish an intravenous adrenaline infusion to treat refractory anaphylaxis (strong recommendation, low certainty evidence).
3.
Low dose intravenous adrenaline infusions appear to be effective and safe to treat refractory anaphylaxis (weak recommendation, very low certainty evidence).
(adapted from RCUK 2008, EAACI 2014 and ASCIA 2020 guidelines, with greater emphasis on early recognition of refractory reactions and further adrenaline treatment, preferably using a low dose IV adrenaline infusion)
Rationale
Around 10% of anaphylaxis reactions (predominantly community reactions to food allergens) have a suboptimal response to a single dose of IM adrenaline, but 98% will respond to 1 or 2 further doses.32 While effective for respiratory symptoms, a single dose of IM adrenaline has a limited effect on reversing the decrease in stroke volume seen during peanut-induced anaphylaxis.51 Case series of refractory anaphylaxis30, 52 and evidence from animal models31, 53 indicate that a poor response to adrenaline is likely due to insufficient adrenaline delivery (a combination of both inadequate dosing with adrenaline, and insufficient circulatory capacity to ensure adequate dose-distribution).
The absorption of adrenaline following IM injection follows a biphasic profile, with the initial peak occurring within 510min.37 International guidelines agree that IM adrenaline should be repeated every 515min where features of anaphylaxis persist;12, 13, 14, 15, 16, 17, 18, 19, 20, 21 the rationale for waiting longer than 5min where symptoms have failed to respond to adrenaline is unclear. In a canine model of anaphylactic shock, a low dose intravenous adrenaline infusion resulted in a far better haemodynamic profile compared to IM or IV bolus treatment.31 Low dose adrenaline infusions are efficacious in case series of human anaphylaxis,30, 54 and are included as the treatment of choice for refractory anaphylaxis in national guidelines in Australia14 and Spain.55 Complications due to adrenaline occur regardless of route but are more common after IV administration, particularly with overly rapid intravenous infusion, bolus administration, and dosing error, for example using 1mg/ml (1:1000) solution (appropriate for IM injection) instead of more dilute solutions e.g. 0.1mg/ml (1:10,000) for intravenous injections.18 These concerns need to be balanced against the risk of death due to refractory anaphylaxis. Reassuringly, appropriate use of low dose intravenous adrenaline infusions appears to both efficacious and safe.30, 54 At least 98% of reactions reported in the literature respond to a maximum of 3 doses of IM adrenaline.32 The working group therefore suggests that following a suboptimal response to 2 doses of adrenaline, expert input is urgently sought to establish a low dose IV adrenaline infusion to provide further vasopressor support (on the basis that this will take at least 5min to set-up, during which a third bolus dose of IM/IV adrenaline should be administered). Given the potential risks of intravenous adrenaline infusion without the necessary expertise and support, and evidence supporting the use of intravenous adrenaline infusions for refractory reactions, we make a strong recommendation that urgent expert support is obtained to establish an intravenous adrenaline infusion to treat refractory anaphylaxis.
With respect to second-line vasopressors, no clear superiority of dopamine, dobutamine, norepinephrine, phenylephrine, or vasopressin (either added to [adrenaline] alone, or compared with one another), has been demonstrated in clinical trials.18 The ASCIA 2020 Guideline recommends consideration of other vasopressors or inotropes only if an IV adrenaline infusion is ineffective.14 Animal models suggest that early treatment with adrenaline followed by continuous adrenaline or vasopressin infusion is superior to vasopressin alone,53, 56 thus confirming that adrenaline must be considered the first-line intervention to treat anaphylactic shock.
Are intravenous fluids effective as an adjuvant treatment for anaphylaxis?
Updated recommendations
1.
In the presence of anaphylaxis with haemodynamic compromise, intravenous (IV) crystalloid fluids should be infused (weak recommendation, very low certainty evidence).
2.
For anaphylaxis refractory to initial treatment with adrenaline, an IV fluid bolus (crystalloid) is recommended as an adjunct to improve drug distribution (weak recommendation, very low certainty evidence).
(adapted from RCUK 2008, EAACI 2014 and ASCIA 2020 guidelines, with addition of fluid bolus to treat refractory reactions even in the absence of obvious haemodynamic compromise)
Rationale
Evidence from observational studies and animal models strongly suggests that anaphylactic shock occurs as a consequence of a profound reduction in venous tone and fluid extravasation. Allergic mediators can also impair cardiac function. This results in a mix of hypovolaemic, distributive and possibly cardiogenic shock, which combine to reduce venous return.57 Guidelines recommend (on the basis of expert consensus) that intravenous fluids are administered to patients with cardiovascular instability, as adrenaline may not be effective without restoring the circulatory volume.1, 12, 14
In peanut-allergic adults, stroke volume was reduced during even mild (non-anaphylaxis) reactions (presumably due to a drop in venous return), although cardiac output was in general maintained due to a compensatory tachycardia.58 A related study in the same cohort found that a single dose of IM adrenaline had limited effect in restoring stroke volume.51 A 5001000mL crystalloid infusion had greater effect in restoring venous return compared to a single dose of IM adrenaline.58 It therefore seems prudent to administer an IV fluid bolus in all cases of anaphylaxis refractory to initial treatment, irrespective of whether there is evidence of haemodynamic compromise. The restoration of circulating volume may aid adrenaline delivery and hasten symptom resolution. A single bolus of IV crystalloid is unlikely to cause overload in the context of anaphylactic shock or refractory anaphylaxis, and judicious use of IV fluids, titrated to clinical response, is potentially lifesaving.
Are antihistamines effective in the treatment of anaphylaxis?
Updated recommendations:
1.
We suggest that antihistamines are not used as part of the initial emergency treatment for anaphylaxis (weak recommendation, low certainty evidence)
-
antihistamines have no role in treating respiratory or cardiovascular symptoms of anaphylaxis
2.
We suggest antihistamines are used to treat skin symptoms which often occur as part of allergic reactions including anaphylaxis (weak recommendation, very low certainty evidence)
-
their use must not delay management of respiratory or cardiovascular symptoms of anaphylaxis (using adrenaline and IV fluids).
(adapted from RCUK 2008, WAO 2011/2020, EAACI 2014 and ASCIA 2020 guidelines, with greater emphasis on the risks of antihistamines delaying timely and appropriate use of adrenaline to treat anaphylaxis)
Rationale
There is no RCT or quasi-RCT evidence to support the use of antihistamines in treating anaphylaxis.1, 12, 21 Antihistamines do not lead to resolution of respiratory or cardiovascular symptoms of anaphylaxis, or improve survival.16, 59, 60 H1-antihistamines cause sedation which can confound symptoms of anaphylaxis,14 and if given by rapid intravenous bolus may precipitate hypotension.1, 12, 61 Recent guidelines relegate antihistamines to a second or third-line intervention; most express a concern that their use can delay the administration of both initial and subsequent doses of adrenaline.1, 12, 14, 16 This is based on a large number of datasets which report that the majority of patients presenting with anaphylaxis to Emergency Departments are treated with antihistamines, yet only a minority receive adrenaline despite an increasing emphasis on adrenaline as the first-line intervention in international guidelines.62, 63, 64, 65, 66, 67, 68 In a large, national prospective registry (Cross-Canada Anaphylaxis Registry, C-CARE), 3498 cases of anaphylaxis were enrolled over a 6 year period; prehospital antihistamine use was associated with a lower rate of administration of >1 adrenaline dose (adjusted OR 0.61; 95% CI 0.440.85), but not other outcomes (hospitalisation/intensive care, intravenous fluids). Moreover, this finding was not robust at sensitivity analyses: excluding less severe reactions, prehospital antihistamine did not affect outcomes; unfortunately, the authors did not assess the impact on >2 doses of adrenaline being given.68 An association between pre-hospital antihistamine use and delayed presentation to healthcare facilities has been reported, resulting in delays in adrenaline administration and increased morbidity.69 Antihistamines do not reduce the occurrence of biphasic reactions.16, 33 An analysis of 9171 anaphylaxis episodes reported to the European Anaphylaxis Register found that antihistamine treatment was significantly associated with the occurrence of biphasic reactions (OR 1.52, 95% CI 1.142.02);34 this may be due to antihistamine use delaying adrenaline administration. We therefore recommend against antihistamines for the acute management of anaphylaxis (weak recommendation); this in consistent with the ASCIA 2020 Guideline.14
Oral H1-antihistamines relieve the cutaneous symptoms of anaphylaxis; combined H1- and H2-antihistamines may be more effective than H1-antihistamines alone, although data are limited.12 However, cutaneous symptoms are not life-threatening and also respond to adrenaline (although the effect may not be long-lasting). The ASCIA 2020 guideline cautions against the use of sedating antihistamines as side effects (drowsiness or lethargy) may mimic some signs of anaphylaxis.14 Antihistamines may be helpful in treating cutaneous symptoms that persist following resolution of anaphylaxis symptoms, but are not recommended until the acute reaction has been successfully treated with more appropriate interventions.1, 12, 13, 14, 15, 16, 17 A non-sedating oral antihistamine is preferred, to avoid confounding due to the risk of sedation which can indicate reaction progression.
Are corticosteroids effective in the treatment of anaphylaxis?
Updated recommendations
1.
We suggest against the routine use of corticosteroids to treat anaphylaxis (weak recommendation, very low certainty evidence).
2.
We suggest corticosteroids may be used as a third line intervention to treat underlying asthma or shock (weak recommendation, very low certainty evidence)
(adapted from RCUK 2008 and JTFPP 2020 guidelines, in view of new data which casts further doubt on the efficacy of steroids to prevent biphasic reactions and possibility of harm (increased need for hospitalisation) in at least one study)
Rationale
The primary action of corticosteroids is the downregulation of the late (rather than early) phase inflammatory response. Given the (slow) absorption kinetics of corticosteroids and their mechanism of action (i.e. through an inhibitory effect on proinflammatory transcription factors such as nuclear factor-B), it is theoretically unlikely that corticosteroids are of benefit in the acute treatment of anaphylaxis;16, 68 the rationale for use is therefore to prevent biphasic reactions. However, a 2012 Cochrane systematic review concluded clinicians should be aware of the lack of a strong evidence base for the use of a glucocorticoid for anaphylaxis.70 Subsequent systematic reviews have confirmed the absence of evidence that corticosteroids reduce reaction severity or prevent biphasic reactions.16, 71
As with antihistamines, corticosteroids are administered far more frequently than adrenaline for the acute treatment of anaphylaxis,62, 63, 64, 65, 66, 67, 68, 70 implying that their use may distract from the need to administer adrenaline. However, of greater concern is emerging evidence that routine use of corticosteroids for anaphylaxis may be harmful, and associated with increased morbidity even after correcting for confounding by indication.68, 72 In the Canadian C-CARE registry, hospitalisation and/or admission to intensive care was associated with prehospital treatment with corticosteroids (OR 2.84; 95% CI 1.556.97, adjusted for reaction severity and treatments administered).68 It is unclear why steroids might increase morbidity: the association was present even after adjusting for prehospital adrenaline.
We therefore recommend against the routine use of corticosteroids to treat anaphylaxis (weak recommendation). Corticosteroids may be of benefit in the following specific scenarios: refractory anaphylaxis (defined as anaphylaxis requiring ongoing treatment despite two appropriate doses of IM adrenaline) and anaphylaxis occurring in the context of poorly-controlled asthma. With the absence of evidence in such cases and the possibility of a different risk:benefit ratio, it is reasonable to include corticosteroids as part of the management for refractory anaphylaxis, but only as an adjunct and not in preference to adrenaline or other inotropes/vasopressor agents.
Are inhaled beta-2 agonists effective in the treatment of anaphylaxis?
Updated recommendation
1.
Beta-2 agonists (such as salbutamol) may be useful as an adjunct treatment for lower respiratory symptoms caused by anaphylaxis, following initial treatment with IM adrenaline (weak recommendation, very low certainty evidence).
2.
In the presence of persisting respiratory symptoms in anaphylaxis, beta-2 agonists (whether inhaled or parenteral) should not be used as an alternative to further parenteral treatment with adrenaline (strong recommendation, very low certainty evidence).
(adapted from RCUK 2008, WAO 2011/2020, EAACI 2014 and ASCIA 2020 guidelines, with greater emphasis on using bronchodilators as an adjunct rather than a replacement for adrenaline)
Rationale
Beta-2 agonists are widely used in clinical practice and feature in most guidelines as a second-line treatment option for anaphylaxis. There is limited evidence to support the use of inhaled beta-2 agonists in the emergency treatment of anaphylaxis and evidence is extrapolated from their use to treat acute asthma.1, 12, 18 International guidelines agree that bronchodilators may be helpful for persisting wheeze, but caution that they do not prevent or relieve upper airway obstruction, hypotension or shock, and should therefore be used as adjunct treatments.1, 12, 14, 17
In patients with mild to moderate respiratory symptoms, beta-2 agonists can be administered by repeated activations of a Metered Dose Inhaler (MDI) via an appropriate large volume spacer where the patient does not require supplementary oxygen. There are insufficient data to make a recommendation over the use of MDIs with spacers in acute severe or life-threatening respiratory symptoms; in these patients, beta-2 agonists should be administered by oxygen-driven nebuliser. There are anecdotal reports of anaphylaxis initially misdiagnosed as severe asthma, which did not respond to parenteral bronchodilator therapy but did respond to adrenaline.73, 74 For this reason, parenteral beta-2 agonists (such as intravenous salbutamol) must not be used in preference to adrenaline for acute anaphylaxis. This recommendation is made on the basis of adrenaline (including further doses) being established as the first-line treatment of anaphylaxis.
How long should patients be observed in hospital following anaphylaxis?
Updated recommendation
We suggest a risk-stratified approach to the discharge of patients following anaphylaxis () (weak recommendation, very low certainty evidence).
Table 5
Suggested observation times following anaphylaxis.
| Consider fast-track discharge (after 2h observation from resolution of anaphylaxis) if: | Minimum 6h observation after resolution of symptoms recommended if: | Observation for at least 12h following resolution of symptoms if any one of the following: |
|---|---|---|
| Good response (within 510 min) to a single dose of adrenaline given within 30min of onset of reaction;AND Complete resolution of symptomsAND The patient already has unused adrenaline auto-injectors (AAI) and has been trained how to use them.AND There is adequate supervision following discharge | 2 doses of IM adrenaline needed to treat reactionaOR Previous biphasic reaction | Severe reaction requiring >2 doses of adrenaline. Patient has severe asthma or reaction involved severe respiratory compromise. Possibility of continuing absorption of allergen e.g. slow release medicines. Patient presents late at night, or may not be able to respond to any deterioration. Patients in areas where access to emergency care is difficult. |
(adapted from RCUK 2008, NICE 2011 and JTFPP 2020 guidelines)
Rationale
The recurrence of anaphylaxis symptoms following initial resolution may be a biphasic reaction but can also represent (and be difficult to distinguish from) protracted anaphylaxis with a transient response to adrenaline, or in the case of food-induced reactions, further allergen absorption from the gastrointestinal tract.75 Historical guidelines have suggested a rate of up to 20% for biphasic reactions, however a recent meta-analysis reported a pooled rate of 4.6% (95% CI 4.05.3).33 A rate of 4.7% has been reported in the European Anaphylaxis Registry.34 In a prospective case series of anaphylaxis presenting to Emergency Departments, delayed deteriorations were noted in 17% (55/315) of reactions, of which 29 (9.2%) required treatment with adrenaline.76
Contradictory ranges for the onset of biphasic symptoms are reported in the literature. The WAO 2011 guideline states that symptoms can recur within 172h (usually within 810h).18 Median times reported in the literature range from 1.7 (Interquartile range 0.74.3) hours76 to 11h i.e. 50% of biphasic reactions began more than 11h after initial symptoms.33 In the European Anaphylaxis Registry, one third of biphasic reactions occurred more than 12h after initial symptoms.34
The optimal duration of observation following anaphylaxis is unknown. The previous RCUK guideline recommended patients should be observed for at least 6h,5 on the basis of data from the UK Fatal Anaphylaxis Register which found that in cases reported to 2000, death never occurred more than 6h after contact with the trigger.77 However, in an updated analysis in 2014, 2.5% of fatalities happened>6h after allergen exposure.29 In 2011, NICE concluded there was no evidence on the effectiveness of observing people or how long people should be observed after a suspected anaphylactic reaction, but in line with RCUK, recommended 612h observation from the onset of symptoms.11 The published literature clearly indicates that this strategy will miss over 50% of biphasic reactions.33, 34, 76 NICE recommends that patients under 16 years should be admitted to hospital under a paediatric team to ensure that children and their parents or carers receive the appropriate care (for example, paediatric assessment, counselling, education) following emergency treatment. However, NICE acknowledges that shorter observation periods could be warranted in those who seek and respond quickly to treatment, particularly in those with a prior diagnosis who already have a management plan and appropriate rescue medication including AAIs.11
The 2020 JTFPP recommends extended observation for patients with severe initial symptoms of anaphylaxis,16 based on a meta-analysis which found biphasic anaphylaxis was associated with a more severe initial presentation (OR 2.11, 95% CI 1.233.61) or administration of>1 dose of adrenaline (OR 4.82, 95% CI 2.708.58). The JTFPP otherwise suggests that 1h observation may be reasonable for low-risk patients with resolved non-severe anaphylaxis; this is supported by a 2019 meta-analysis which reported that 1h observation would capture 95.0% (95%CI 99.097.3%) of biphasic reactions.78 Extending this interval would only impact slightly on the rate of biphasic reactions captured: 96.5% (95%CI 93.498.2%) for 4h, 97.3% (95%CI 95.098.5%) for 6h and 98.2% (95%CI 96.799.1%) for 12h observation. Prolonged observation is inconvenient for many patients (and their carers), and is not cost-effective for patients at low risk of biphasic reactions.79
After considering the available evidence, the working group was concerned that the previous RCUK recommendation might offer false reassurance in terms of mitigating against the risk of biphasic reaction. To balance the risks and benefits involved, we instead propose a pragmatic, risk-stratified and individualised approach to determining the length of observation following anaphylaxis ().
Discussion
In general, the certainty of evidence underpinning anaphylaxis management is low or very low. The GRADE-ADOLOPMENT process provides a robust and transparent mechanism to assess the current evidence for treatment of anaphylaxis, to inform the 2021 RCUK Anaphylaxis Guideline update. A strength of this approach is that it should reduce discordance between different guidelines, and highlight the reasons for any discrepancies. Through a public consultation, we were able to include responses from key stakeholders, ensuring that our recommendations considered the values and preferences of clinicians, patients and carers. We have previously commented anaphylaxis is anaphylaxis, irrespective of where it occurs: it does not vary in presentation or response to treatment depending on country or region. As a community, we need to achieve an international consensus on what we do know, and transparency over those areas for which (at best) there is limited evidence and at worst, emerging data that such interventions may do harm.80 We hope this evidence review serves as an initial step in this process.
Conclusion
We used the GRADE-ADOLOPMENT process to evaluate current evidence for the emergency treatment of anaphylaxis, incorporating a public consultation, to inform the updated 2021 Resuscitation Council UK Anaphylaxis Guideline.
Conflicts of interest
J. Soar is joint-chair of the Anaphylaxis Working group of the UK Resuscitation Council, Editor of Resuscitation and receives payment from the publisher Elsevier. P.J. Turner is supported by a UK Medical Research Council Clinician Scientist award (reference MR/K010468/1) and reports grants from UK Medical Research Council, NIHR/Imperial Biomedical Research Centre, UK Food Standards Agency, End Allergies Together, and Jon Moulton Charity Trust; personal fees and nonfinancial support from Aimmune Therapeutics, DBV Technologies, and Allergenis; personal fees and other fees from ILSI Europe and UK Food Standards Agency, outside the submitted work; and is current Chairperson of the WAO Anaphylaxis Committee, Chairperson of the Paediatric Allergy Group of the British Society for Allergy and Clinical Immunology, and joint-chair of the Anaphylaxis Working group of the UK Resuscitation Council. A.F. Whyte is current Chairperson of the Adult Allergy Group of the British Society for Allergy and Clinical Immunology. The rest of the authors declare that they have no relevant conflicts of interest.
CRediT authorship contribution statement
Amy Dodd: Methodology, Analysis, Writing - original draft, Writing - review & editing.
Anna Hughes: Methodology, Analysis, Writing - original draft, Writing - review & editing. Nicholas Sargant: Methodology, Analysis, Writing - review & editing. Andrew F Whyte: Methodology, Analysis, Writing - review & editing. Jasmeet Soar: Conceptualization, Methodology, Analysis, Writing - review & editing, Project administration. Paul J. Turner: Conceptualization, Methodology, Analysis, Writing - original draft, Writing - review & editing, Project administration.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We are grateful to the following individuals for providing internal review to the updated RCUK Anaphylaxis Guideline and the updated recommendations. Sophie Farooque, Adam Fox, Graham Roberts, Hazel Gowland (patient advocate); on behalf of Resuscitation Council UK: Charles Deakin, Joe Fawke, David Gabbott, Matt Griffiths, Andrew Lockey, Ian Maconochie, Jerry Nolan, Gavin Perkins, Sophie Skellett.
Appendix A.Supplementary data
The following are Supplementary data to this article:
References
2.
Panesar S.S., Javad S., de Silva D. EAACI Food Allergy and Anaphylaxis Group. The epidemiology of anaphylaxis in Europe: a systematic review. Allergy. 2013;68:13531361. [PubMed] [Google Scholar]4.
McLure M., Eastwood K., Parr M., Bray J. A Rapid review of advanced life support guidelines for cardiac arrest associated with anaphylaxis. Resuscitation. 2021;159:137149. [PubMed] [Google Scholar]5.
Soar J., Pumphrey R., Cant A. Working Group of the Resuscitation Council (UK). Emergency treatment of anaphylactic reactions - guidelines for healthcare providers. Resuscitation. 2008;77:157169. [PubMed] [Google Scholar]6.
Schnemann H.J., Wiercioch W., Brozek J. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101110. [PubMed] [Google Scholar]7.
Alonso-Coello P., Schnemann H.J., Moberg J. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. [PubMed] [Google Scholar]8.
Andrews J., Guyatt G., Oxman A.D. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719725. [PubMed] [Google Scholar]9.
Krishna M.T., Ewan P.W., Diwakar L. Diagnosis and management of hymenoptera venom allergy: British Society for Allergy and Clinical Immunology (BSACI) guidelines. Clin Exp Allergy. 2011;41:12011220. [PubMed] [Google Scholar]10.
Ewan P., Brathwaite N., Leech S. BSACI guideline: prescribing an adrenaline auto-injector. Clin Exp Allergy. 2016;46:12581280. [PubMed] [Google Scholar]12.
Muraro A., Roberts G., Worm M. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:10261045. [PubMed] [Google Scholar]15.
Campbell R.L., Li J.T., Nicklas R.A., Sadosty A.T. Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113:599608. [PubMed] [Google Scholar]16.
Shaker M.S., Wallace D.V., Golden D.B.K. Anaphylaxis a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145:10821123. [PubMed] [Google Scholar]18.
Simons F.E.R., Ardusso L.R.F., Bilo M.B. World Allergy Organization guidelines for the assessment and management of anaphylaxis. J Allergy Clin Immunol. 2011;127:593.e1-e22. [PMC free article] [PubMed] [Google Scholar]19.
Simons F.E., Ardusso L.R., Bil M.B. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol. 2012;12:389399. [PubMed] [Google Scholar]20.
Simons F.E., Ardusso L.R., Dimov V. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013;162:193204. [PubMed] [Google Scholar]21.
Simons F.E., Ebisawa M., Sanchez-Borges M. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8:32. [PMC free article] [PubMed] [Google Scholar]22.
Dreskin S.C., Halsey N.A., Kelso J.M. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9:32. [PMC free article] [PubMed] [Google Scholar]23.
de Silva D., Singh C., Muraro A. European Academy of Allergy and Clinical Immunology Food Allergy and Anaphylaxis Guidelines Group. Diagnosing, managing and preventing anaphylaxis: systematic review. Allergy. 2020 doi:10.1111/all.14580. [PubMed] [CrossRef] [Google Scholar]24.
Ko B., Kim J., Seo D.W. Should adrenaline be used in patients with hemodynamically stable anaphylaxis? Incident case control study nested within a retrospective cohort study. Sci Rep. 2016;6:20168. doi:10.1038/srep20168. [PMC free article] [PubMed] [CrossRef] [Google Scholar]25.
Umasunthar T., Leonardi-Bee J., Hodes M. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43:13331341. [PMC free article] [PubMed] [Google Scholar]26.
Nassiri M., Babina M., Dlle S. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol. 2015;135:491499. [PubMed] [Google Scholar]27.
Noimark L., Wales J., Du Toit G. The use of adrenaline autoinjectors by children and teenagers. Clin Exp Allergy. 2012;42:284292. [PubMed] [Google Scholar]28.
Grabenhenrich L.B., Dlle S., Ruff F. Epinephrine in severe allergic reactions: the European Anaphylaxis Register. J Allergy Clin Immunol Pract. 2018;6 18981906.e1. [PubMed] [Google Scholar]29.
Pumphrey R., Sturm G. Risk factors for fatal anaphylaxis. In: Moneret-Vautrin D.A., editor. Advances in anaphylaxis management. Future Medicine; London: 2014. pp. 3248. [Google Scholar]30.
Brown S.G., Blackman K.E., Stenlake V., Heddle R.J. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J. 2004;21:149154. [PMC free article] [PubMed] [Google Scholar]31.
Mink S.N., Simons F.E., Simons K.J., Becker A.B., Duke K. Constant infusion of epinephrine, but not bolus treatment, improves haemodynamic recovery in anaphylactic shock in dogs. Clin Exp Allergy. 2004;34:17761783. [PubMed] [Google Scholar]32.
Patel N., Chong K.W., Yip A.Y.G. Use of multiple epinephrine doses in anaphylaxis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2021;(April) doi:10.1016/j.jaci.2021.03.042. S0091-6749(21)00566-2, Epub ahead of print. [PMC free article] [PubMed] [CrossRef] [Google Scholar]33.
Lee S., Bellolio M.F., Hess E.P., Erwin P., Murad M.H., Campbell R.L. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015;3 40816.e1-2. [PubMed] [Google Scholar]34.
Kraft M., Scherer Hofmeier K., Ruff F. Risk factors and characteristics of biphasic anaphylaxis. J Allergy Clin Immunol Pract. 2020;8 33883395.e6. [PubMed] [Google Scholar]35.
Turner P.J., DunnGalvin A., Hourihane J.O. The emperor has no symptoms: the risks of a blanket approach to using epinephrine autoinjectors for all allergic reactions. J Allergy Clin Immunol Pract. 2016;4:11431146. [PMC free article] [PubMed] [Google Scholar]36.
Liu X., Lee S., Lohse C.M., Hardy C.T., Campbell R.L. Biphasic reactions in emergency department anaphylaxis patients: a prospective cohort study. J Allergy Clin Immunol Pract. 2020;8:12301238. [PubMed] [Google Scholar]38.
Campbell R.L., Bellolio M.F., Knutson B.D. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3:7680. [PubMed] [Google Scholar]39.
Macdougall C.F., Cant A.J., Colver A.F. How dangerous is food allergy in childhood? The incidence of severe and fatal allergic reactions across the UK and Ireland. Arch Dis Child. 2002;86:236239. [PMC free article] [PubMed] [Google Scholar]42.
Simons F.E., Chan E.S., Gu X., Simons K.J. Epinephrine for the out-of-hospital (first-aid) treatment of anaphylaxis in infants: is the ampule/syringe/needle method practical? J Allergy Clin Immunol. 2001;108:10401044. [PubMed] [Google Scholar]43.
Simons FE, Gu X, Silver NA, Simons KJ. EpiPen Jr versus EpiPen in young children weighing 15 to 30kg at risk for anaphylaxis. J Allergy Clin Immunol 109:1715. [PubMed]44.
Patel N., Isaacs E., Duca B. What dose of epinephrine? Safety and pharmacokinetics of 0.5mg versus 0.3mg epinephrine by autoinjector in food-allergic teenagers: a randomized cross-over trial. J Allergy Clin Immunol. 2020;145:AB6. [Google Scholar]45.
Duvauchelle T., Robert P., Donazzolo Y. Bioavailability and cardiovascular effects of adrenaline administered by anapen autoinjector in healthy volunteers. J Allergy Clin Immunol Pract. 2018;6:12571263. [PubMed] [Google Scholar]47.
Suwan P., Praphaiphin P., Chatchatee P. Randomized comparison of caregivers ability to use epinephrine autoinjectors and prefilled syringes for anaphylaxis. Asian Pac J Allergy Immunol. 2018;36:248256. [PubMed] [Google Scholar]48.
Asch D., Pfeifer K.E., Arango J. Benefit of epinephrine autoinjector for treatment of contrast reactions: comparison of errors, administration times, and provider preferences. AJR Am J Roentgenol. 2017;209:W363W369. [PubMed] [Google Scholar]51.
Turner P.J., Ruiz-Garcia M., Durham S.R., Boyle R.J. Limited effect of intramuscular epinephrine on cardiovascular parameters during peanut-induced anaphylaxis: An observational cohort study. J Allergy Clin Immunol Pract. 2021;9 527530.e1. [PMC free article] [PubMed] [Google Scholar]53.
Dewachter P., Rath-Fries I., Jouan-Hureaux V. A comparison of epinephrine only, arginine vasopressin only, and epinephrine followed by arginine vasopressin on the survival rate in a rat model of anaphylactic shock. Anesthesiology. 2007;106:977983. [PubMed] [Google Scholar]54.
Alviani C., Burrell S., Macleod A. Anaphylaxis Refractory to intramuscular adrenaline during in-hospital food challenges: a case series and proposed management. Clin Exp Allergy. 2020;50:14001405. [PubMed] [Google Scholar]55.
Cardona V., Cabaes N., Chivato T. 2016. Spanish Society of Allergology and Clinical Immunology (SEAIC), Gua de actuacin en ANAFILAXIA: GALAXIA. [CrossRef] [Google Scholar]56.
Zheng F., Collange O., Davidson J. Epinephrine, compared with arginine vasopressin, is associated with similar haemodynamic effects but significantly improved brain oxygenation in the early phase of anaphylactic shock in rats: an experimental study. Eur J Anaesthesiol. 2015;32:563570. [PubMed] [Google Scholar]57.
Brown S.G. The pathophysiology of shock in anaphylaxis. Immunol Allergy Clin North Am. 2007;27:165175. [PubMed] [Google Scholar]58.
Ruiz-Garcia M., Bartra J., Alvarez O. Cardiovascular changes during peanut-induced allergic reactions in human subjects. J Allergy Clin Immunol. 2020 doi:10.1016/j.jaci.2020.06.033. [PMC free article] [PubMed] [CrossRef] [Google Scholar]59.
Dhami S., Panesar S.S., Roberts G. Management of anaphylaxis: a systematic review. Allergy. 2014;69:168175. [PubMed] [Google Scholar]60.
Nurmatov U.B., Rhatigan E., Simons F.E., Sheikh A. H2-antihistamines for the treatment of anaphylaxis with and without shock: a systematic review. Ann Allergy Asthma Immunol. 2014;112:126131. [PubMed] [Google Scholar]61.
Ellis B.C., Brown S.G. Parenteral antihistamines cause hypotension in anaphylaxis. Emerg Med Australas. 2013;25:9293. [PubMed] [Google Scholar]62.
Huang F., Chawla K., Jrvinen K.M., Nowak-Wgrzyn A. Anaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomes. J Allergy Clin Immunol. 2012;129 162-8.e1-3. [PMC free article] [PubMed] [Google Scholar]63.
Beyer K., Eckermann O., Hompes S., Grabenhenrich L., Worm M. Anaphylaxis in an emergency setting elicitors, therapy and incidence of severe allergic reactions. Allergy. 2012;67:14511456. [PubMed] [Google Scholar]64.
Fineman S.M. Optimal treatment of anaphylaxis: antihistamines versus epinephrine. Postgrad Med. 2014;126:7381. [PubMed] [Google Scholar]65.
Ruiz Oropeza A., Lassen A., Halken S., Bindslev-Jensen C., Mortz C.G. Anaphylaxis in an emergency care setting: a one year prospective study in children and adults. Scand J Trauma Resusc Emerg Med. 2017;25:111. [PMC free article] [PubMed] [Google Scholar]66.
Dubus J.C., L M.S., Vitte J. Use of epinephrine in emergency department depends on anaphylaxis severity in children. Eur J Pediatr. 2019;178:6975. [PubMed] [Google Scholar]67.
Choi Y.J., Kim J., Jung J.Y., Kwon H., Park J.W. Underuse of epinephrine for pediatric anaphylaxis victims in the emergency department: a population-based study. Allergy Asthma Immunol Res. 2019;11:529537. [PMC free article] [PubMed] [Google Scholar]68.
Gabrielli S., Clarke A., Morris J. Evaluation of prehospital management in a Canadian Emergency Department Anaphylaxis Cohort. J Allergy Clin Immunol Pract. 2019;7 2232-2238.e3. [PubMed] [Google Scholar]69.
Wiley E., Romo N. The association of antihistamine administration and delayed presentation for care in pediatric patients admitted with anaphylaxis. Pediatrics. 2020;146:236238. [Google Scholar]70.
Choo K.J., Simons F.E., Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Cochrane Database Syst Rev. 2012:CD007596. doi:10.1002/14651858.CD007596.pub3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]71.
Alqurashi W., Ellis A.K. Do corticosteroids prevent biphasic anaphylaxis? J Allergy Clin Immunol Pract. 2017;5:11941205. [PubMed] [Google Scholar]72.
Campbell D.E. Anaphylaxis management: time to re-evaluate the role of corticosteroids. J Allergy Clin Immunol Pract. 2019;7:22392240. [PubMed] [Google Scholar]74.
Payus A.O., Ibrahim A., Mustafa N. Two Stones on One Bird: a case report on severe biphasic anaphylaxis masquerading as life-threatening acute asthma. Open Access Maced J Med Sci. 2018;6:21362138. [PMC free article] [PubMed] [Google Scholar]75.
Turner P.J., Ruiz-Garcia M., Patel N., Abrantes G., Burrell S., Vazquez-Ortiz M. Delayed symptoms and Orthostatic Intolerance following peanut challenge. Clin Exp Allergy. 2021;51(5):696702. [PubMed] [Google Scholar]76.
Brown S.G., Stone S.F., Fatovich D.M. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013;132:11411149. [PubMed] [Google Scholar]77.
Pumphrey R.S. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:11441150. [PubMed] [Google Scholar]78.
Kim T.H., Yoon S.H., Hong H., Kang H.R., Cho S.H., Lee S.Y. Duration of observation for detecting a biphasic reaction in anaphylaxis: a meta-analysis. Int Arch Allergy Immunol. 2019;179:3136. [PubMed] [Google Scholar]79.
Shaker M., Wallace D., Golden D.B.K., Oppenheimer J., Greenhawt M. Simulation of health and economic benefits of extended observation of resolved anaphylaxis. JAMA Netw Open. 2019;2:e1913951. [PMC free article] [PubMed] [Google Scholar]80.
Dodd A., Hughes A., Turner P.J. Anaphylaxis management why are guidelines inconsistent? A rapid review of advanced life support guidelines for cardiac arrest associated with anaphylaxis. Resuscitation. 2021;159:165167. [PubMed] [Google Scholar]